Marshall syndrome is a fairly common condition in childhood, but its description in Russian literature is few. It is believed that Marshall's syndrome is an autoinflammatory disease of unknown etiology. It is characterized by episodes of fever lasting 3-6 days with recurrence every 3-8 weeks, associated with at least one of three main symptoms: aphthous stomatitis, cervical lymphadenitis and pharyngitis, in 15% of cases, exanthema syndrome is noted. The first letters of the symptom complex (periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis) it was called "PFAPA-syndrome". The debut of the disease usually falls in the age of up to 5 years and, is spontaneously resolved in adolescence.
What Immunologic Mechanisms Are Involved in PFAPA Flares?
It is not improbable that MEFV and/or NLRP3 variants are involved in the pathogenesis of PFAPA, since they are functionally linked to inflammatory reactions taking place during PFAPA flares. Protein products of both of these genes regulate activation of caspase-1, which processes proIL-1β and proIL-18 to mature forms . IL-1β and IL-18 are important mediators of the inflammatory response leading to fever, elevated acute-phase proteins, and neutrophilia . It has been associated with the pathogenesis of the majority of hereditary periodic fever syndromes and recently also with PFAPA syndrome [3].
Stojanov et al. showed that fever attacks in 6 PFAPA patients led to significant increase in serum concentrations of IL-1β and other proinflammatory cytokines (IL-6, IFNγ, and TNF-α) when compared to healthy controls (11 children) . Brown et al. also reported increases in IL-6 sera concentrations during PFAPA flares, whereas levels of IL-1β, TNF-α, and IFNγ remain low [1,2] . Similar results were presented in a Norwegian study comparing 22 PFAPA patients and 14 children with pneumonia . As the time after the onset of fever, in which sera were drawn, was the shortest in Stojanov et al.'s study (6–12 hours) , it was presumed that IL-1β and TNF-α peak early in the fever period and then quickly approach homeostatic levels. This was not confirmed by Kubota et al., who found increased levels of IL-1β and TNF-α in blood collected from 9 PFAPA patients within 96 hours from fever onset. In a recent study among 33 patients from Japan IL-1β and TNF-α were not elevated, while IL-18, IL-6, and IFNγ increased during PFAPA febrile attacks[5,9]. Two other studies failed to find elevated levels of IL-1β in PFAPA patients; nevertheless, indirect evidence of the increased IL-1β production during PFAPA fever attacks was discovered. Stojanov et al. reported overexpression of IL-1-related and inflammasome-related genes, increased sera concentrations of IL-1β-induced proinflammatory cytokines (IL-6 and G-CSF), and, finally, prompt clinical response to IL-1 receptor antagonist treatment (anakinra) in 5/5 PFAPA patients. Kolly et al. found increased levels of caspase-1 and significantly higher secretion of IL-1β by stimulated peripheral blood mononuclear cells during febrile episodes compared to afebrile periods[4,3].
Increase in serum concentrations of IL-18 was demonstrated in several studies. IL-18 stimulates release of IFNγ that was also elevated in the serum of PFAPA patients as well as IFNγinducible chemokines.
Elevated levels of IL-1β, IL-18, IL-6, and IFNγ point to innate immunity dysregulation as the key pathomechanism of PFAPA attacks. The concept is advocated by other immunological aberrations during PFAPA flares such as monocytosis, increased count and characteristic features of neutrophils ,increased secretion of proinflammatory chemokines (IP10/CXCL10, MIG/CXCL9), and upregulated transcription of complement genes[10].
Increased neutrophil and monocyte counts during febrile episodes have been reported in several PFAPA cohorts. Furthermore, Stojanov et al. found significant alteration of neutrophil functions during PFAPA flares: diminished rates of spontaneous apoptosis, increased generation of intracellular NADPH oxidase-derived ROS (Reactive Oxygen Species), and signatures of priming such as granule mobilization and receptor upregulation of the cell surface[7].
Chemokines are induced directly by early innate response mechanisms and under the influence of IFNγ. They are strong chemoattractants of T-cells to the inflammation sites and may act as a link between innate and adaptive immune responses, which in PFAPA is mainly Th1-driven[]. Th1-induced elevation of IFNγ and CXCL10 levels in the absence of the increased Th2- and Th17-cytokines weighs heavily in favour of Th1-type inflammatory response in PFAPA. The suppression of Th2 type response is supported by low IL-4 levels and low expression of IL-4 gene in peripheral blood and in the tonsils. Additionally, lack of eosinopenia has been linked to Th2-driven inflammation ,whereas several studies reported eosinopenia in PFAPA patients during febrile episodes, which also argues in favour of Th1-driven immune response in PFAPA syndrome[2].
Involvement of adaptive immunity in PFAPA pathogenesis is indicated by fluctuations in T lymphocytes and increase in serum IFNγ levels during the attack periods. A decrease in the number of circulating lymphocytes during PFAPA flares was found in several studies, two of which reported a decrease in both CD4+ and CD8+ lymphocyte counts. As previously suggested, this may result from activation and recruitment of T-cells to peripheral tissues and is clinically reflected by tonsillitis and cervical adenitis[6]. Two recently published studies support this hypothesis. Yamazaki et al. showed, that neutrophils and monocytes of PFAPA patients (n = 33) during attacks strongly expressed CD64, an Fcγ receptor, which might be upregulated by IFNγ, possibly from retention of activated T-cells in the periphery. A study by Petra et al. revealed increased levels of CD8+ T-cells, the transitional B cells, and naive stages of both the CD4+ and CD8+ T-cells in the tonsils from PFAPA patients (n = 10), compared to tonsils from children with obstructive sleep apnea syndrome (n = 10)[5] .
The studies exploring pathophysiology of PFAPA differed in terms of characteristics and size of the PFAPA cohort, presence and type of control group, examined proteins, and laboratory methods. Most of them support the hypothesis of an abnormal, IL-1β dependent innate immune response to an environmental trigger that leads to Th1-driven inflammation, expressed by recruitment of T-cells to periphery[3].
In summary, PFAPA is an autoinflammatory disease of compound, heterogeneous etiology. It shares some clinical and pathogenic features with monogenic recurrent fever syndromes; however, studies have failed to identify its genetic basis. Possible involvement of MEFV and NLRP mutations is in line with their functional connection to IL-1β dependent innate inflammatory response, which might be involved in PFAPA pathogenesis. Most autoinflammatory diseases derive from genetic variants of the innate immune system, and except for TRAPS, all monogenic periodic syndromes belong to IL-1β-regulated autoinflammatory diseases [1]. However, the pathophysiology of PFAPA syndrome seems to be more complex. Inflammatory response in PFAPA is also driven by Th1-type adaptive immune response, which also dominates in several autoimmune diseases, for example, Hashimoto's thyroiditis, Grave's disease, Crohn's disease, psoriasis, Type 1 diabetes, and Rheumatoid arthritis. Th1-driven immune response mainly develops following infections by viruses, intracellular bacteria, and parasites, while IL-1βactivation has been shown to be involved in bacterial, viral, and fungal infections. The role of a viral or other infectious agents in PFAPA etiology is unknown, though the hitherto published studies are pointing to collaboration of environmental and immunological factors in a genetically inclined individual.
Criteria for diagnosis of Marshall’s syndrome in children
(K.T. Thomas, 1999)
1. Recurrent episodes of fever with regular intervals, beginning at the age of 5 years
2. Symptoms of general disorders in the absence of signs of acute respiratory disease plus one of the following clinical signs:
v aphthous stomatitis;
v cervical lymphadenitis;
v tonsillitis (pharyngitis).
3. Markers of acute inflammatory process during the febrile episode (at least 1):
v leukocytosis;
v increased ESR.
4. Complete absence of symptoms between febrile episodes
5. Normal growth and development of the child
The aim of the study was to inform the target audience that PFAPA-syndrome, being one of the causes of recurrent fever of unclear genesis in childhood, occurs much more often than it is diagnosed.
In this report, we give our own observation of a child with Marshal's syndrome. Participation in the study was accompanied by the mandatory signing of a protocol of voluntary informed consent by the patient's parents.
Bolna G., Armenian, 2 years 11 months entered the infectious disease department of the CSTO in Stavropol with complaints of a fever of 39.00C, a decrease in appetite, a rash on the palms and feet, a specific smell from the mouth.
From the history of the disease it is known that the child fell ill 4 days ago, when there was an increase in body temperature to 38.5-39.00C 2-3 times a day, for 3 days. The patient received symptomatic and local treatment. According to the mother, the girl has been ill since January 2016 every month. According to the outpatient card data, the first episode was recorded on January 15, 2016, she was observed outpatiently with a diagnosis: acute nasopharyngitis; received: flemoxin-solutab, anaferon, panavir, symptomatic treatment. February 8, 2016, fell ill again, was treated at a pediatric site with a diagnosis of lacunar angina, received: amoxiclav, symptomatic therapy. 02.03.2016 - acute nasopharyngitis (arbidol, influferon, panavir, kipferon, symptomatic treatment). 05.03.2016 - lacunar angina (flemoclav solutab) was diagnosed again, a ring-shaped rash on the palms and soles was first observed. The rash was regarded as allergic, and received antihistamines. 27.04.2016. - the next episode of lacunar angina, received an antibiotic from the group of cephalosporins. 06.05.2016 - acute nasopharyngitis, acute obstructive bronchitis (inhalation therapy). 05/20/2016 - infectious mononucleosis: tonsillopharyngitis protracted course (ceftriaxone, antiviral therapy), 03.08.2016 - bacterial infection protracted course (chemomycin, interferonotherapy). With repeated studies in the general analysis of blood - the tendency to neutropenia.
From the social history it is known that the child is from the 1st pregnancy, which occurred without pathology. Delivery was urgent, at 39-40 weeks by caesarean section, early discharge of amniotic fluid, pelvic presentation of the fetus; Body weight - 3500 g, body length - 52 cm, head circumference - 36 cm. APGAR- 8-9 points. Breast fed within the 1st day. Received Mixed feeds up to 4 months, then artificial feeds introduced at the appropriate time. Physical and neuropsychic development correspond to age. At age 1 she was registered with a neurologist with the diagnosis: Perinatal encephalopathy, motor disorders syndrome, hypertensive hydrocephalic syndrome. Vaccinated on an individual schedule (no R1 DTP, R2 poliomyelitis, against pneumococcal infection not vaccinated for medical reasons). Postponed diseases: SARS (2-3 times a year until January 2016), food allergy (9 months), vulvitis.
When examined by the department, the condition was of medium severity, the skin was pale, warm to touch, elastic, moderate humidity, "periorbital shadows", a ring-shaped rash 3-5 cm in diameter of bright pink color was visible on the sole of the right foot (Fig. Peripheral lymph nodes: posterior cervical to 0.5-0.7 cm, submandibular to 1.5-1.7 cm, on both sides, elastic, painless on palpation, not soldered with underlying tissues. Nasal breathing is free, periodic snoring, there was no discharge. In the oropharynx - the tongue is moist, clean. There is hyperemia of the palatine arch, tongue, posterior pharyngeal wall, tonsils. On the tonsils- filamentous incrustations white in color. No noted pathology in the respiratory and cardiovascularsystems.
Additional investigations: CBC- WBC 6.06x109 / L, ESR - 30 mm / h; CRP - 54.7 units; consultation of ENT-doctor - chronic tonsillitis; consultation of a rheumatologist: a recurring viral-bacterial infection. Risk group for vasculitis, Behcet's disease, cyclic neutropenia.
The child received symptomatic treatment, was discharged on the 10th day with recovery. After 4 days after discharge, a repeated increase in temperature to febrile digits, the appearance of a rash on the feet (Figure 1), in the popliteal region (Fig. 2). On the mucosa of the tooth-gingival fold, in the region of the lower anterior incisors – aphtha 0.5 cm in diameter (Fig. 3). Tonsils hypertrophied, loose with a filamentous coating.

Fig.1. Patient G., 2 years 11 months. Diagnosis: Marshall syndrome (ring-shaped rash)
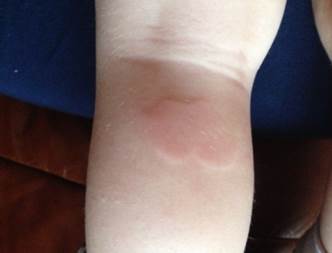
Fig.2. Patient G., 2 years 11 months. Diagnosis: Marshall syndrome (urticaria rash).
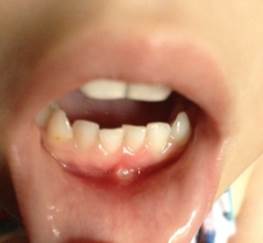
Fig.3. Patient G., 2 years 11 months. Diagnosis: Marshall syndrome (aphthae on the oral mucosa).
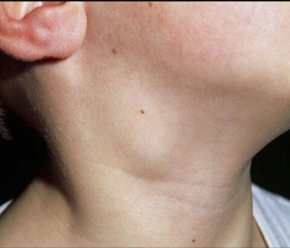
Fig.4 Patient G., 2 years 11 months. Diagnosis: Marshall syndrome (enlargement of submandibular lymph nodes).
Given the clear periodicity of fever attacks with a characteristic symptom complex, inflammatory changes in blood tests during the febrile seizure, the lack of effect from antibacterial and antipyretic therapy, the Marshall syndrome was suspected. Prednisolone test was not carried out. Therapy with glucocorticosteroids is non-specific and complicates differential diagnosis with other auto-inflammatory diseases (consultations in the RCCH were planned). In connection with the lack of accepted medical indications for tonsillectomy, the operation was not performed.
Considering the need for a differential diagnosis with Behcet's disease, cyclic neutropenia, Familial Mediterranean Fever, the girl was sent to the RCCH in Moscow.
Conclusion: Given the medical history, clinical presentation and laboratory data, the diagnosis of Marshall Syndrome (PFAPA-syndrome) is most likely. Recommended: to carry out a genetic examination - searching for mutations in the MEFV gene with the aim of excluding Familial Mediterranean Fever (FMF), taking into account the Armenian roots of the child, bilateral adenotonsillectomy. In a stable condition, she was discharged to continue treatment on an outpatient basis.
The awareness of pediatricians and ENT doctors about PFAPA-syndrome is important for timely diagnosis of this disease, evaluation of its prognosis and development of joint treatment tactics that will ultimately improve the quality of life of children with Marshall syndrome, allows: coping with acute episodes of the disease, tonsillectomy, and avoids unreasonable antibiotic therapy.
Библиографическая ссылка
Федько Н.А., Воронкина Е.Н., Рубачева О.Е., Джегру С.Т., Грэхем С.В. СИНДРОМ МАРШАЛЛА .КЛИНИЧЕСКИЙ СЛУЧАЙ . // Международный студенческий научный вестник. – 2017. – № 6. ;URL: https://eduherald.ru/ru/article/view?id=17905 (дата обращения: 21.11.2024).

